Life History & Behaviour
As a highly specialised predator, it has been speculated that P. gardineri feeds on invertebrates such as flatworms or polychaete worms (Gosliner 1981; Marshall & Willan 1999). It has however, been discovered that once they feed on any invertebrates that produce toxins, they transform these toxins internally to create their own toxic form of mucus (Thompson 1960).
Rudman 1972 looked at the gut function within Aglajidae and more specifically those species with in the genus Aglaja which are synonymous with Philinopsis. Algajids are active feeders which feed on active prey as they have a large muscular buccal bulb (Rudman 1972b). In these animals, there are two short salivary glands that are attached to the posterior region of the buccal bulb (Lobo-da-Cunha et al. 2009). Once it has left the buccal bulb, the oesophagus widens to form a distensible crop where prey digestion is initiated (Rudman 1972b). In the crop, the soft tissues of the prey are dissolved and the nutritive fluid travels to the small stomach that is connected to the digestive gland which is where nutrient absorption mostly occurs (Rudman 1972b).
As the shell of P. gardineri has been reduced and internalised over years of evolution and adaptation to its soft-sediment environment, it suggests that this organism uses other defensive strategies in order to protect itself from predators and the environment. In the case of opisthobranch molluscs, the chemical defence they used resides within the selective concentration of chemicals that their food contains (Marin & Ros 2004). The secondary metabolites they produce are generated via the propionate route in its glandular structures and are quite unusual in nature, however are commonly found in marine molluscs (Marin & Ros 2004).

In terms of reproduction, a study by Anthes and Michiels (2007) looked at the reproductive life history of several species including P. gardnineri. P. gardnineri is hermaphroditic and displays exclusively simultaneous reciprocal insemination as shown in figure 1. They found that copulation time is on average 60.5 minutes with ±SD of 60.12 and that body size is proportional to the amount of eggs produced indicating that they invest in quantity rather than quality (Anthes & Michiels 2007). They produce balloon-shaped egg masses, relatively large in diameter, that are attached to the substrate with a fine mucus thread that they produce (Anthes & Michiels 2007). The capsules that hold the eggs are arranged in clearly visible strands and it usually takes 134 hours ±SD of 49 hours for the eggs to develop (Anthes & Michiels 2007).
Picture: Harry Blalock
Burrowing Capacity of Philinopsis gardineri
Introduction:
P. gardineri is an Opisthobranch that inhabits coral reefs on the reef flat area among the sand. Very little is known about this species due to its inconspicuous nature and discreet life history as it spends a lot of its time burrowed beneath the sand. When above sand it is suspected that is conducting a search pattern in order to find prey. The aim of this study is to determine the burrowing capacity in different sand grain sizes. It is hypothesised that P. gardineri will burrow the easiest in medium sized grain of sand as it is not too big that it is difficult for it to push past and not too small that it has difficulty binding mucus to it in order to burrow.
Methods:
One specimen of P. gardineri was collected from Heron Island (23°26S, 151°51E) which is a coral cay that has been vegetated over time within the Capricorn-Bunker Island group, located at the lower end of the Great Barrier Reef. P. gardineri was collected in the area near the reef crest before being transported back to Heron Island Research Station, being held in aquarium tanks.
Three different types of sand were collected on the beach:
1. Large grain
2. Medium grain
3. Small grain
For each type of sand, 8 repetitions were made using the one individual. For each repetition, burrowing time was recorded with a stopwatch from the beginning of burrowing until the animal stopped moving and was at least half covered in sand.
Results:
This experiment shows that interestingly, the medium grain sand took the longest burrowing time and coarse and fine sand took reasonably similar times. Standard deviation was lowest in the coarse sand indicating that it took similar amounts of times during repetition compared to medium and fine grain sizes which are more variable in the times.
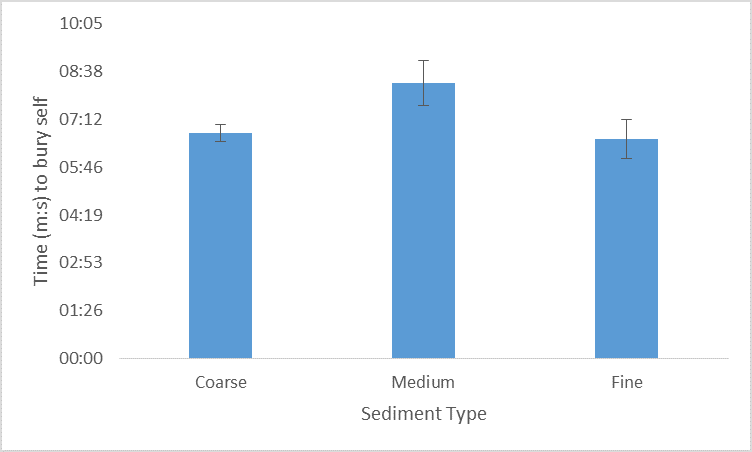
Discussion:
The hypothesis for this study has not been supported as P. gardineri took the longest to burrow in the medium grain sediment. However, this is not indicative of all organisms of P. gardineri, as only one organism was used during this experiment. It is however, suggestive that coarse and fine sediments can be burrowed into with relative ease despite the different grain sizes.
|